Abstract
Background: Patients with cancer treated with immune checkpoint inhibitors (ICI) have a substantial risk of venous thromboembolism (VTE). Conventional prothrombotic risk factors seem inefficient in the prediction of VTE under ICI-therapy. Recently, C-reactive protein (CRP) trajectories were proposed as prognostic and predictive biomarker for survival and therapy response in patients treated with ICI. The aim of the present study was to explore early dynamics of systemic CRP-levels after initiation of ICI for the prediction of VTE.
Methods: Adults patients with cancer treated with ICI at the Medical University of Vienna were included in this retrospective cohort study. Levels of CRP were obtained at study baseline within 4 weeks prior to and longitudinally at 1, 2, and 3 months after ICI-initiation. Patients were stratified according to their CRP-dynamics relative to baseline measurements, defining a CRP-flare as an increase by the factor of 2.5 relative to baseline measurements and a CRP-response as a 50% relative decrease in CRP-levels within the first 3 months after ICI-initiation, respectively. Patients were followed for the time of ICI therapy, the initiation of any subsequent anti-cancer therapy, death, or a maximum of 3 months after the last ICI-cycle. Risk of VTE was analysed in a competing-risk framework, accounting for all-cause mortality as competing outcome event.
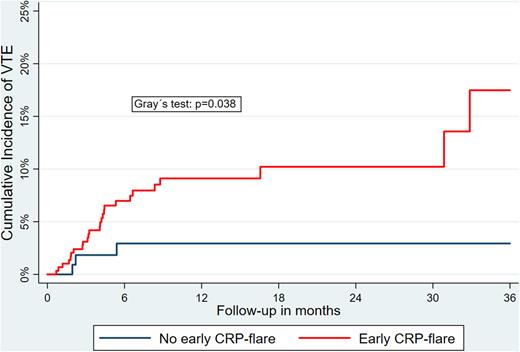
Results: Overall, 405 patients treated with ICI were included (median age: 63 years [interquartile range, IQR: 53-72]; 38% female). The most frequent tumour types were melanoma (33%) and lung cancer (26%), with 91% of patients diagnosed with stage IV cancer at ICI-initiation. Over a median follow-up of 7.9 months (IQR: 4-16), we observed 29 VTE (cumulative incidence: 12.7% [95% confidence interval, CI: 6.9-20.5]). In patients with a CRP-flare after ICI-initiation (n=296; 73%), an increased risk of VTE was observed (sub-distribution hazard ratio (SHR): 3.58 [95%CI: 1.07-11.94], p=0.038), which prevailed upon multivariable adjustment for tumour type, cancer stage, age, sex, Charlson comorbidity index, and ECOG-performance status. Cumulative VTE-incidences in patients with a CRP-flare were 17.5% (95%CI: 8.8-28.7), compared to 2.9% (95%CI: 0.8-7.7) in the remainder of patients (Figure 1). Further, in patients with a CRP-response within the first 3 months after therapy initiation (n=126; 31%), numerically lower VTE rates were observed compared to the remainder of patients (SHR: 0.41 [95%CI: 0.16-1.06], p=0.066), with the lowest VTE-rates in those with CRP-responses without a prior CRP-flare (n=62; 15%; cumulative VTE-incidence: 1.6% [95%CI: 0.1-7.6]).
Conclusion: Early dynamics of systemic CRP-levels are associated with risk of VTE during ICI-therapy, with the highest rates observed in patients with an initial CRP-flare. These data suggest a potential link between the ICI-induced systemic inflammatory response and subsequent risk of VTE.
Figure 1: Cumulative incidence of VTE according to early CRP-flare after ICI-initiation. Competing risk cumulative incidence, accounting for all-cause mortality as competing outcome event. CRP-flare was defined as increase in CRP-levels by the factor of 2.5 within 3 months after ICI-initiation relative to baseline, pretherapeutic measurements.
Disclosures
Höller:Amgen, BMS, MSD, Novartis, and Roche: Honoraria; Amgen, Astra Zeneca, BMS, Inzyte, MSD, Novartis, Pierre Fabre, and Roche: Membership on an entity's Board of Directors or advisory committees. Fuereder:MSD, Merck Darmstadt, Roche, BMS, Accord, Sanofi, and Boehringer Ingelheim: Honoraria; MSD, Merck Darmstadt, Amgen, Pfizer, and Sanofi: Membership on an entity's Board of Directors or advisory committees. Jost:Abbvie: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Ariad: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Astra Zeneca: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel/accomodation; Bayer: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; BMS/Celgene: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel/accomodation, Research Funding; Boehringer: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel/Accomodation, Research Funding; Janssen (Johnson and Johnson): Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel.accomodation; MSD: Other: Travel/accomodation; Novartis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel/accomodation, Research Funding; Pfizer: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel/accomodation; Pierre Fabre: Other: Travel/Accomodation; Roche: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel/accomodation, Research Funding; Servier: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees. Pabinger:Bayer AG, Boehringer Ingelheim, Daiichi Sanchyo, and BMS/ Pfizer: Honoraria, Membership on an entity's Board of Directors or advisory committees. Preusser:Bayer, Bristol Myers Squibb, Novartis, Gerson Lehrman Group (GLG), CMC Contrast, GlaxoSmithKline, Mundipharma, Roche, BMJ Journals, MedMedia, Astra Zeneca, AbbVie, Lilly, Medahead, Daiichi Sankyo, Sanofi, Merck Sharp & Dome, and Tocagen: Honoraria, Membership on an entity's Board of Directors or advisory committees. Ay:Bayer, Boehringer Ingelheim, Daiichi Sankyo, and BMS/Pfizer: Membership on an entity's Board of Directors or advisory committees; Bayer, Daiichi Sankyo, BMS/Pfizer, and Sanofi: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal